Bridge the gap between quality and operational teams with no mental effort to save up to 60% of your time
Ask the smart assistant to find information you look for in all existing PDFs/Scans/Word to get it instantly
Maintain all the versions and track all product and company updates in one hand.
Keep all your information at one place. Transfer any type of file (paper scans/PDFs/Word/Excel etc.) instantly to manage all the informations with one click
Accelerate your internal approval process across the entire organization by e-signatures and automated approval flows.
Automated User Management: Define your process once—tasks auto-assign to the right roles and people.
Simplified Documentation: Reports are pre-filled with product and company data to make documentation fast and effortless.
Bridge Between Teams: Connect your operations and QMS teams to ensure alignment on shared knowledge.
Interactive Process Modeler: All to-dos and forms in one place—with smart reminders.
Forms Manager: Share structured data in any format and auto-generate forms from records.

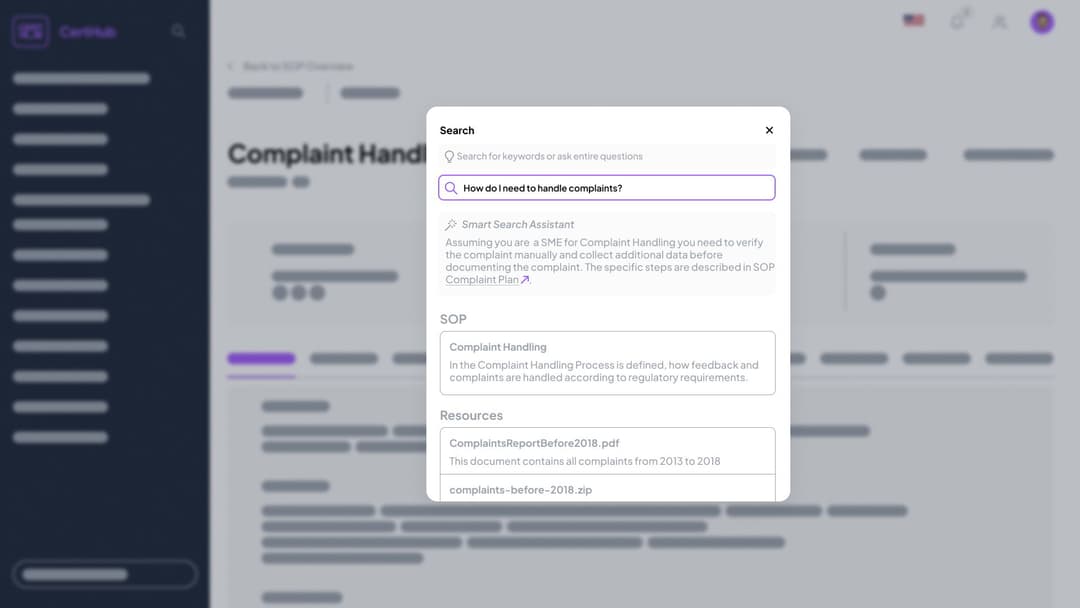
Comprehensive Search: Find exactly what you need across all data—just ask in the search bar.
Quick Overview: Instantly see clear summaries of the information you need.
State of Art Data Security: Use AI confidently—your data stays secure at every step.
Direct Navigation: Go straight to the right place to continue working on detailed tasks.
Version Control: Maintain continuous version control for all changes and updates.
Proof of Evolution: Clearly demonstrate your product’s journey through development and maintenance.
Always be Audit-Ready: Easily jump to the versions you need and demonstrate compliance during audits.



All-in-One File Management: Manage and search all documents including legacy documents—even scanned papers—on a single platform.
Hybrid File Upload: Upload any structured/unstructured file—CertHub transforms PDFs, Excels, scans, and papers into structured data.
No Redundancy: Skip parallel updates—CertHub keeps everything synced in one place.
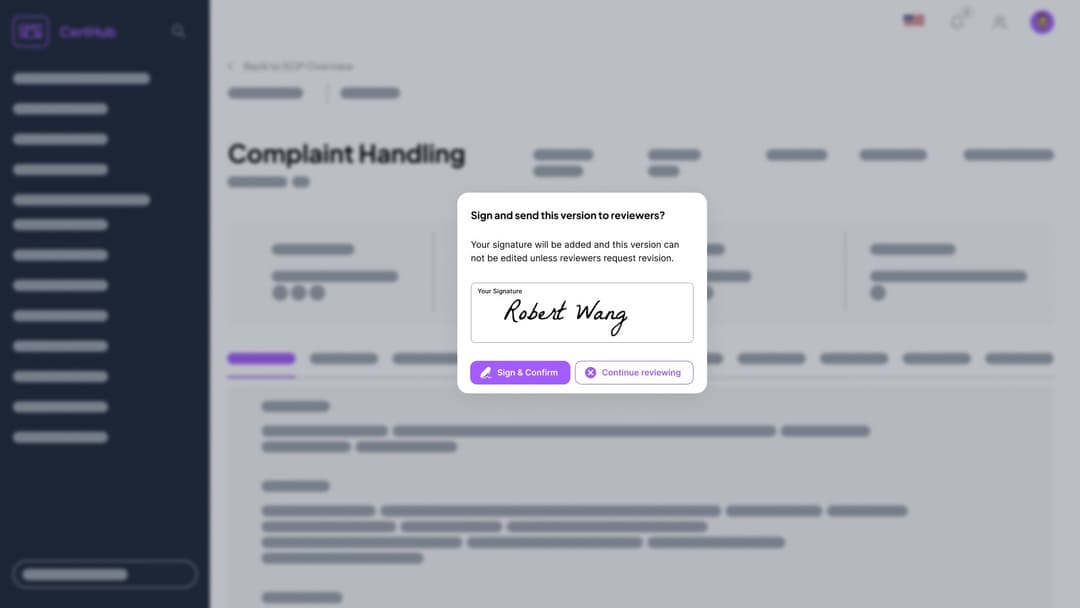
Automated Notifications: CertHub automatically notifies colleagues when it's their turn to sign.
Digital E-Signature: Sign SOPs, instructions, and records with one click—anytime, anywhere.
Secure & Compliant: Ensure secure, compliant signatures for all processes.
Role-Based Flexibility: Keep processes moving—others in the role can sign during absences.

Automated User Management: Define your process once—tasks auto-assign to the right roles and people.
Simplified Documentation: Reports are pre-filled with product and company data to make documentation fast and effortless.
Bridge Between Teams: Connect your operations and QMS teams to ensure alignment on shared knowledge.
Interactive Process Modeler: All to-dos and forms in one place—with smart reminders.
Forms Manager: Share structured data in any format and auto-generate forms from records.

Comprehensive Search: Find exactly what you need across all data—just ask in the search bar.
Quick Overview: Instantly see clear summaries of the information you need.
State of Art Data Security: Use AI confidently—your data stays secure at every step.
Direct Navigation: Go straight to the right place to continue working on detailed tasks.

Version Control: Maintain continuous version control for all changes and updates.
Proof of Evolution: Clearly demonstrate your product’s journey through development and maintenance.
Always be Audit-Ready: Easily jump to the versions you need and demonstrate compliance during audits.

All-in-One File Management: Manage and search all documents including legacy documents—even scanned papers—on a single platform.
Hybrid File Upload: Upload any structured/unstructured file—CertHub transforms PDFs, Excels, scans, and papers into structured data.
No Redundancy: Skip parallel updates—CertHub keeps everything synced in one place.

Automated Notifications: CertHub automatically notifies colleagues when it's their turn to sign.
Digital E-Signature: Sign SOPs, instructions, and records with one click—anytime, anywhere.
Secure & Compliant: Ensure secure, compliant signatures for all processes.
Role-Based Flexibility: Keep processes moving—others in the role can sign during absences.
© CertHub 2024